Atomic Structure (Physics)
- Thomson's tried to explain about the arrangement of +ve & -ve charges in an atom.
- An atom is a +vely charged sphere of diameter about 10m is which +ve charge are uniformly distributed where as -ve charges are slightly embedded like plum in a pudding.
- This model is also called plum-pudding model.
- He explained unequal concentration of electric change.
- Atom is electrically neutral, the concentration of electric charge must be equal.
- This model could not explain about the atomic stability.
- This model could not explain about -particle scattering experiment.
Hence, this model is fails to exist
Rutherford's carried out of particle scattering experiment and achieved the following observation.
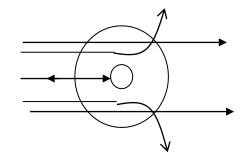
Observation
- Most of -particles cross thin gold foil without any deflection.
- Some of -particles are deflected with particular angle i.e. less than 90i.e. acute angle.
- Few -particles are totally back at an angle 180.
Postulates
- The +vely charged and massive particles are concentrated to the inner core of an atom that inner core is called nucleus having diameter about 10m to 10m.
- The space around the nucleus is totally empty.
- The -vely charged particles are revolving round the circular path, known as electrons.
Failures
- This model could not explain atomic stability.
- This model could not explain origin of line spectra.
Therefore, Rutherford's model fails to exist
Application of Rutherford model is to determine the closed distance of approach
When +vely charged particle approaches towards a stationary nucleus then due to repulsion between them the kinetic energy of +vely charged particle gradually decreases & a stage come at which potential energy gain by charged particle due to stationary nucleus balanced its K.E.
We have,
Closest distance
The closest distance of approach of any +vely charged particle to the stationary nucleus.
- Electron revolves round the stationary orbit. The necessary centripetal force provided by the electrostatic force of attraction between electron & nucleus.
i.e - Those orbits are called stationary orbits in which an angular momentum of an e is integral multiple of h/2.
i.e. - Electrons absorbs energy to jump from lower orbit to higher orbit and when an electron jump from higher energy state to lower energy state, it will radiate energy.
i.e
Radiated wavelength is: - The no of possible photons due to transition of electron from energy level are:
where, is the no. of orbit.
Radius of orbit
The radius of n
for so,
If
if and so on.Velocity of in orbit
Velocity of in orbit of atom is:
We have,
For hydrogen atom . So,
If
If
& so on.Energy in orbit
Total energy in orbit is:
Again,
For hydrogen , So,Time period for an in the orbit
For hydrogenFrequency of an in the orbit
For hydrogen So,
Eqn for wavelength is,
Where,
lower energy state
higher energy stateCurrent :
Magnetic field at the center is :
Lyman Series:
If
Then
For line
so,
for series limit
so,
It lies in u-v regionBalmer series:
If
So,
For line
so,
for series limit
so,
It lies in visible regionPaschen series:
If
So,
For line
so,
for series limit
so,
It lies in IR regionBrackett series:
If
So,
For line
so,
for series limit
so,
It lies in IR region
Amount of energy required to jump the electrons from ground state to higher energy state is known as excitation energy. Required potential to do so is called excitation potential.
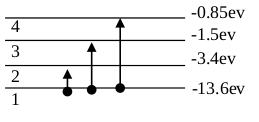
to
Example:
excitation energy is
Excitation potential
Amount of energy required to jump the electrons from any energy state to infinite energy state is known as inonization energy. Required potential to knock out the electrous from an atom is known as ionization potential.

When an ionized from
Requried ionization energy is:
ionization potential
And so on.
Heisenberg's uncentainty principle states that the product of uncertainity of two conjugate variables is always greater than or equal to .
i.e.
position
uncertainity is position
Momentum
uncentainity is momentum
Similary for energy & time
ie.
- He explained dual nature of matter (wave nature of matter)
- If matter posses wave nature, it will be associated particular wavelength ie
h = planck's constant
p = momentum
Let,
m = mass of matter
V = velocity of matter
Therefore, de Broglie wavelength for matter is: - When an is accelerated through p.d. of V then de-Broglie's wavelength for an electron is:
P.E. gain by
Accelerated
We have P.E = K.E
We have, de-Broglie wave length for an electron is: